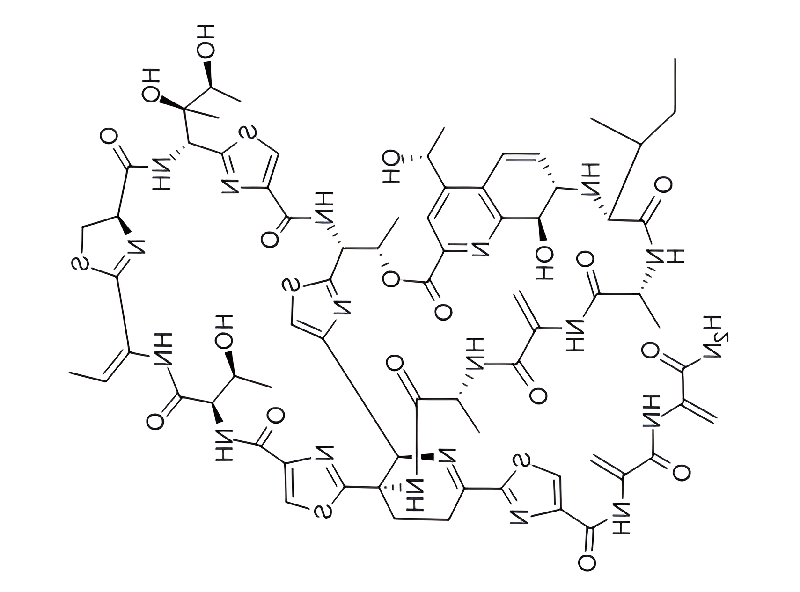
Thiostrepton is an extremely complex natural bacterial product that is used as a topical veterinary antibiotic and also has good antimalarial and anticancer activity. Currently, it is completely chemically synthesized.
Thiostrepton, first isolated from bacteria in 1955, has unusual antibiotic activity: it inhibits protein biosynthesis by binding to ribosomal RNA and its associated proteins. Dorothy Crowfoot Hodgkin, a British crystallographer and 1964 Nobel Prize winner, discovered the structure in 1970.
Thiostrepton contains 10 rings, 11 peptide bonds, extensive unsaturation, and 17 stereocenters. Even more challenging is the fact that it is very sensitive to acids and bases. It is the parent compound and the most complex member of the thiopeptide antibiotic family.
Now this compound has succumbed to the synthetic sweet talk of chemistry professor K.S. Nicolaou and his colleagues from the Scripps Research Institute and the University of California at San Diego [Angew. Chem. internationality. Editors, 43, 5087 and 5092 (2004)].
Christopher J. Moody, Senior Research Fellow in the Department of Chemistry at the University of Exeter, UK, commented: “This is a landmark synthesis and a remarkable achievement by the Nicolaou group.” doxorubicin D.
Key to the structure of THIOSTREPTON is the dehydropiperidine ring, which supports the didehydroalanine tail and two macrocycles – a 26-membered thiazoline-containing ring and a 27-membered quinalcolic acid system. Nicolaou and colleagues created the key dehydropiperidine ring from simple starting materials using a biomimetic iso-Diels–Alder dimerization reaction. This important step helped confirm the 1978 proposal that bacteria use this reaction to biosynthesize thiopeptide antibiotics.
Nicolaou and colleagues incorporated dehydropiperidine into a thiazoline-containing macrocycle. They combined this macrocycle with a structure containing quinalcolic acid and a didehydroalanine tail precursor. They then purified the product to obtain thiostrepton.
Reviewers of the group’s two papers said the synthesis “is a masterpiece that highlights state-of-the-art technologies and opens new horizons for meaningful research into structure, activity, and mode of action.”
Post time: Oct-31-2023